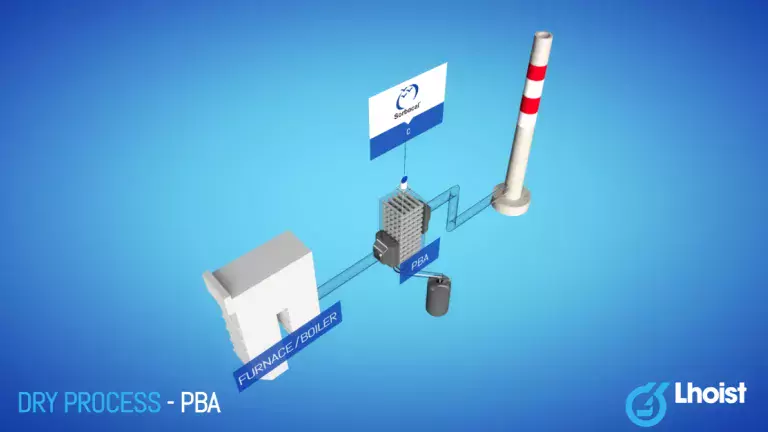
Packed bed absorber (PBA)
What is Packed Bed Absorber (PBA) process?
In the PBA process flue gas passes in a cross- or counter-flow through a reaction chamber filled with a granular sorbent. Gravity draws the reagent through the reaction chamber, after which it is removed at the bottom of the PBA. The removal performance of a conventional PBA using natural limestone is quite high for SO3 and HF (> 95-98%) but rather limited for HCl (20-30%) and SO2 (10-20%).
A packed bed reactor consists of a vessel filled with reactive material. While passing through the vessel, the pollutants are captured by the filler. The filler needs to be replaced after a certain time, which varies from plant to plant.
Benefits
The PBA is particular suitable for industrial processes with a peak release of acidic pollutants. Sorbacal® HCG significantly increases the removal performance for HCl and SO2. This makes it an attractive alternative in PBA processes for new applications, like the desulphurization of marine diesel engines.
Lhoist product range dedicated to PBA
Sorbacal® C, a natural limestone CaCO3 sorbent in the form of “chippings”, is typically used for PBAs.
For greater removal performance we recommend Sorbacal® G: spherical granulated particles consisting of CaCO3 and Ca(OH)2. The residual Ca(OH)2 content in combination with the higher porosity and surface area of the granulates enables significantly better removal rates for HCl (> 70%) and SO2 (30-35%). With multi-stage PBAs, even higher rates have been achieved for HCl and SO2 (>80%).
- Sorbacal® C
- Sorbacal® G