Alumina
Alumina applications
Lime is used in various process steps during the refining process to convert bauxite ore into alumina oxide product in high purity powder form.
Alumina oxide product (Al2O3) in high purity powder form, which is used as a feed source for aluminium metal smelters.
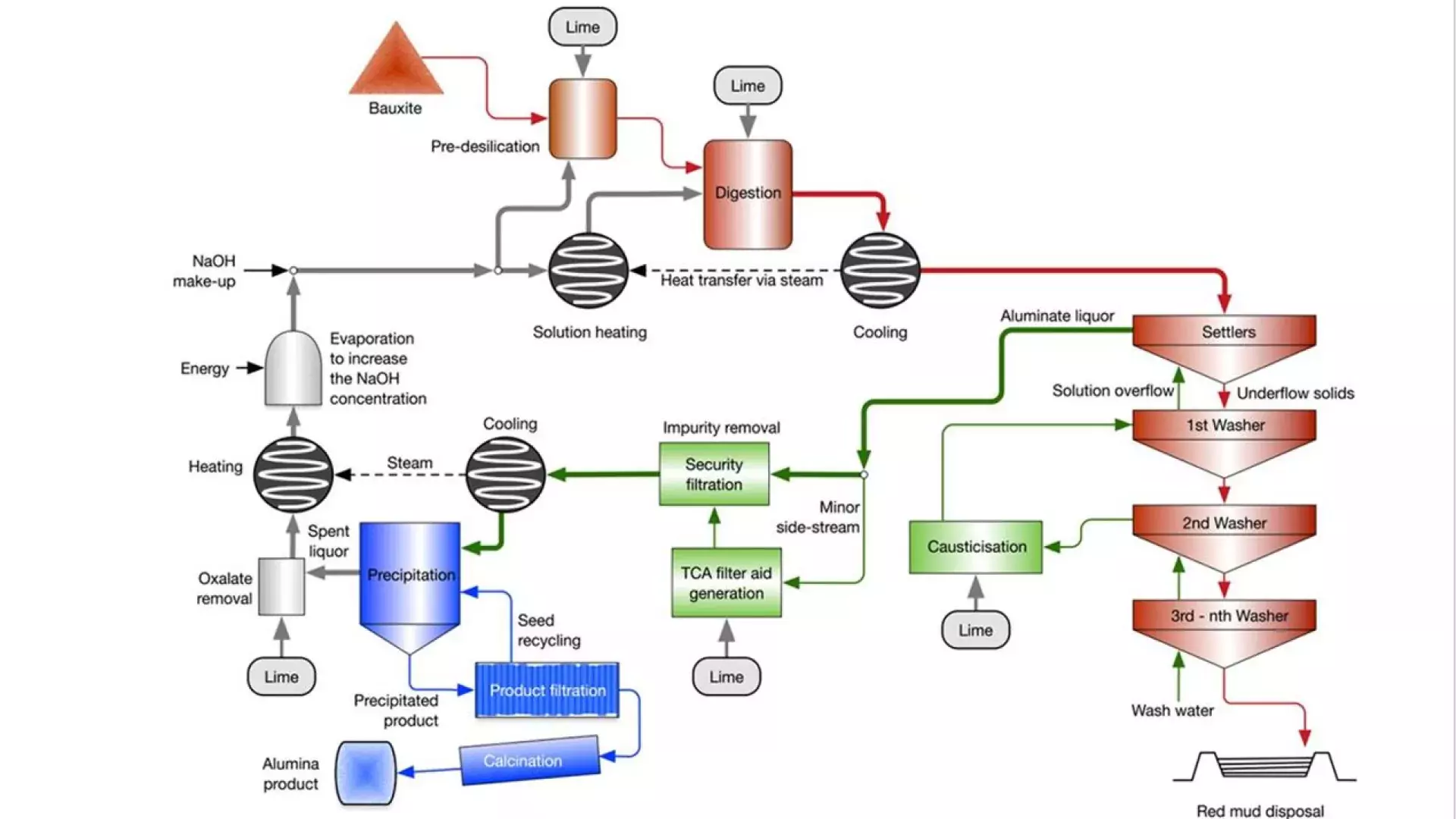
Digestion
During digestion of aluminium-containing bauxite ore, sodium hydroxide (NaOH), also called caustic, is used to dissolve (or digest) the ore at high temperature and pressure conditions to solubilize alumina as soluble sodium aluminate. A portion of silica minerals also dissolve and reprecipitate as sodalite, also known as desilication product (DSP). This precipitation process is important because it removes silica from the solution.
However, the precipitation of silica as sodalite also results in the loss of sodium (Na+), that cannot be regenerated as NaOH. To counter this loss of sodium, some plants add slaked lime to digestion where Ca(OH)2 causes dissolved silica to precipitate as hydrogarnet, which contains calcium rather than sodium, thereby reducing the loss of sodium to silica precipitates.
Causticization
NaOH used in digestion is converted into Na2CO3 due to reactions with organic material contained in the bauxite ore and because of the reaction with CO2 from contact with air. Too high Na2CO3 concentrations are detrimental to alumina dissolution.
Ca(OH)2 is used to convert Na2CO3 into NaOH, in a causticization reaction, thereby reducing Na2CO3 and sustaining a sufficiently high NaOH concentration in digestion to avoid precipitation of alumina.
Filter aid
Alumina Bayer refineries make use of filtration to remove fine (<5 micron) suspended particulate impurities, not removed by gravity settling in thickeners. Filtration occurs prior to alumina (Al2O3) precipitation and aims to remove fine iron and silica particles.
The filtration process is enhanced by so-called “filter aid” particles consisting of tri-calcium aluminate (Ca3[Al(OH)6]2), produced by reacting a small side-stream of the high-strength process liquor (at 95 – 105°C), containing sodium aluminate NaAl(OH)4, with Ca(OH)2 added as milk of lime / slaked lime slurry.
Oxalate removal
Bauxite ore feed contains several organic components, due to the shallow nature of bauxite ore deposits. In the Bayer process most organics eventually degrade to oxalate.
Oxalate is the most refractory organic compound and is an important contaminant in Bayer process refineries. Some plants use slaked lime to control the oxalate content in the circuit by precipitation as calcium oxalate (CaC2O4.H2O).